2018
A Disintegrin and Metalloproteases (ADAMs): Activation, Regulation and Mechanisms of Catalysis
The aim of this Special Issue of IJMS is to provide novel insights into the physiological and pathological functions of these membrane-anchored molecular scissors. To this end, we welcome experts in the field to contribute research articles and critical reviews on molecular mechanisms involved in the regulation of these fascinating enzymes.

2017
Immunology and Immune-Based Disease Treatment Retreat
We attended the 6th annual retreat of the Center for Immunology and Immune-Based Disease in Iowa City on August 17, 2017
6th Annual Immunology and Immune-Based Disease Treatment Retreat
Read more...
August 17th, 2017
Tumor Necrosis Factor α (TNFα) is a potent pro-inflammatory cytokine that plays a crucial role in the underlying pathogenesis of chronic inflammatory systemic diseases such as rheumatoid arthritis, obesity-induced low-grade inflammation, and inflammatory bowel disease. The success of biologics that inhibit TNFα, such as adalimumab, etanercept, and infliximab, for treatment of chronic inflammation has led to a desire for orally available small molecules that have fewer serious adverse events and are more cost efficient to produce than current TNFα antagonists.
An attractive target for anti-TNF therapy therefore is the TNFα-converting enzyme, TACE, also known as a disintegrin and metalloproteinase 17 (ADAM17), which promotes the release of soluble TNFα from its membrane-bound precursor. We have recently identified a novel regulator of TACE, a catalytically inactive member of the rhomboid family of intra-membrane proteinases called iRhom2. Mice lacking iRhom2 are unable to release TNFα from their immune cells and are therefore protected from inflammatory arthritis. The main goal of studies in our lab is to identify new therapeutic targets in chronic inflammatory disorders by using mouse models of human disease, which can provide mechanistic insights into the disease process as well as access to all disease stages.
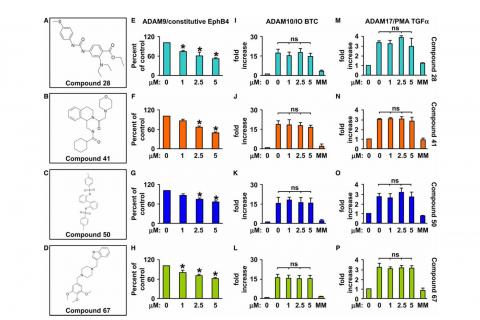
Publication: "Characterization of the catalytic properties of the menbrane-anchored metalloproteinase ADAM9 in cell-based assays."
Here we report that ADAM9-dependent shedding of EphB4 is not stimulated by three commonly employed activators of ADAM-dependent ectodomain shedding: phorbol esters, pervanadate or calcium ionophores.
Our Most Recent Paper Published in The Biochemical Journal
Read more...
Here we report that ADAM9-dependent shedding of EphB4 is not stimulated by three commonly employed activators of ADAM-dependent ectodomain shedding: phorbol esters, pervanadate or calcium ionophores. With respect to the inhibitor profile, we show that ADAM9 is inhibited by the hydroxamate-based metalloprotease inhibitors marimastat, TAPI-2, BB94, GM6001 and GW280264X, and by 10 nM of the tissue inhibitor of metalloproteinases (TIMP)-3, but not by up to 20 nM of TIMP-1 or-2. Additionally, we screened a non-hydroxamate small-molecule library for novel ADAM9 inhibitors and identified four compounds that selectively inhibited ADAM9-dependent proteolysis over ADAM10- or ADAM17-dependent processing. The identification of novel nonhydroxamate inhibitors of ADAM9 could provide the basis for designing more selective compounds that block the contribution of ADAM9 to pathological neovascularization and cancer.
2016
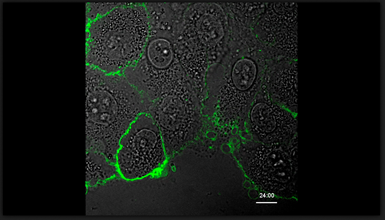
Phosphatidylserine exposure is required for ADAM17 sheddase function
pSIVA live cell imaging of HaCaT keratinocyte stimulated with ionomycin (IO). IO induces rapid PS exposure spreading over the entire cell surface and remains sustained over the time of observation.
2013
iRHOM2 is a critical pathogenic mediator of inflammatory arthritis
Video from: J Clin Invest. 2013 Feb;123(2):928-32. doi: 10.1172/JCI66168. Epub 2013 Jan 25.
Researchers Identify New Target for Rheumatoid Arthritis Drugs
Priya Issuree and Thorsten Maretzky found that iRhom2 regulates TACE on immune cells, whereas iRhom1 is responsible for helping TACE mature elsewhere in the body, such as in brain, heart, kidney, liver, lung and spleen cells.
