Research sections
Regulation of ADAM proteases
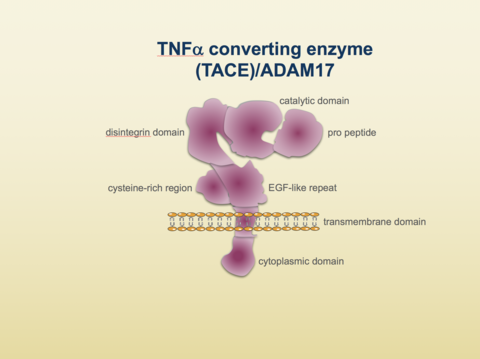
"A typical ADAM (a disintegrin and metalloprotease) protein has the following domain organization: the extracellular domain contains an N-terminal metalloprotease domain, a disintegrin domain, a cysteine-rich region and an epidermal growth factor (EGF)-like domain; the cytoplasmic domain frequently contains signaling motifs such as phosphorylation sites or proline-rich regions. ADAMs also contain an N-terminal signal sequence and a pro-domain.”
“Activation of ADAM17 involves a rapid and reversible exposure of its catalytic site. It rapidly responds to the physiological signaling pathways stimulated by thrombin, EGF, lysophosphatidic acid and TNFα. Stimulation of ADAM17 is swift and quickly reversible, and does not depend on removal of its inhibitory pro-domain by pro-protein convertases, or on dissociation of an endogenous inhibitor, TIMP3. Moreover, activation of ADAM17 by physiological stimuli requires its transmembrane domain, but not its cytoplasmic domain.”
Nat Rev Mol Cell Biol. 2005 Jan;6(1):32-43. (edited)
J Cell Sci. 2010 Nov 15;123(Pt 22):3913-22. doi: 10.1242/jcs.069997. Epub 2010 Oct 27. (edited)
iRhoms in disease and development
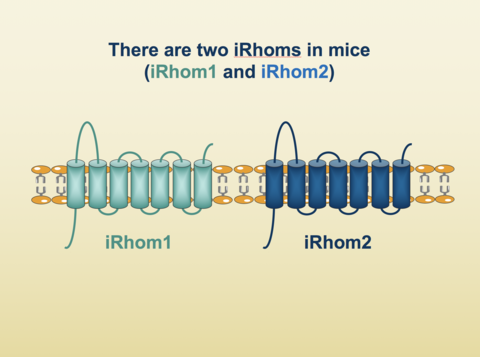
“There are two iRhoms. iRhom1 appears to have broad functionality, whereas iRhom2 has more restricted and exclusive functions. iRhoms are proteolytically inactive homologs of rhomboid proteases. They localize to the membrane of the ER and were initially shown to be part of the ER protein quality control machinery both in Drosophila and mammalian cells. iRhom2 is mediating the release of TNFα from macrophages. iRhom2 acts as a cargo receptor that binds TNFα convertase in the ER and helps TACE to get to the plasma membrane. There, TACE cleaves the membrane-bound TNF-α precursor, resulting in release of the soluble TNF-α cytokine. Consequently, genetic inactivation of iRhom2 blocks TACE activity at the cell surface of macrophages and other immune cells, largely preventing TNF-α release. TNFα acts as key pathologic player in inflammatory diseases, such as rheumatoid arthritis, sepsis, and psoriasis, but is also required for innate immunity. An impairment of TNFα production has been described in iRhom2-deficient mice, making them resistant to LPS-induced septic shock, although they succumb more rapidly to bacterial infection.“
TNF signaling & Rheumatoid Arthritis
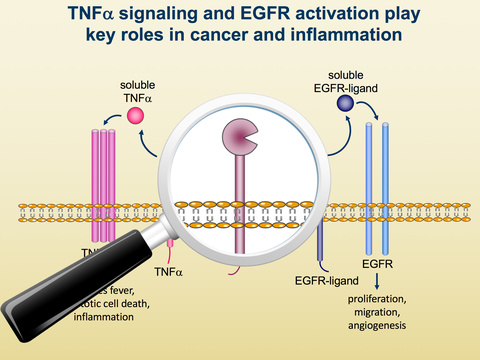
“TNF-α convertase is an essential regulator of TNF activity and, as such, has been considered as a potential therapeutic target for rheumatoid arthritis (RA). However, as TACE is not only required for the shedding of soluble TNF from cells, but also for activating epidermal growth factor receptor (EGFR) signalling (important for maintenance of skin and intestinal integrity), concerns over adverse effects resulting from blocking EGFR activation have hampered this approach. IRhom2 regulates the activity of TACE and is critical for the development of inflammatory arthritis in mice and could represent a new drug target for RA.”
Nature Reviews Rheumatology 9, 136 (March 2013) | doi:10.1038/nrrheum.2013.19 (edited)
EGFR activation & Cancer
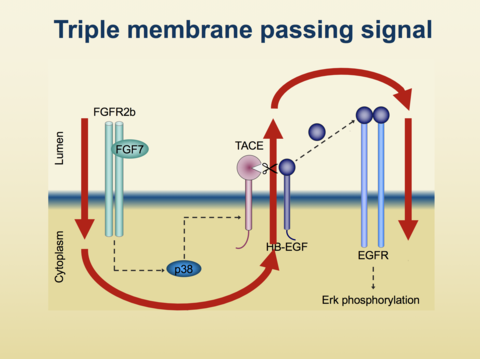
“Binding of FGF7 to FGFR2b stimulates TACE in a manner that can be blocked by inhibitors of Src kinases, p38 MAP-kinase and of phosphatidylinositol 3 kinase, which is responsible for the conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-trisphosphate. Stimulation of ADAM17 by FGF7 requires its transmembrane domain, but not the cytoplasmic domain. Activation of ADAM17 triggers release of membrane proteins that include the EGFR-ligand HB-EGF, which activates EGFR and ERK1/2, thereby promoting FGF7-stimulated cell migration in keratinocytes. Both FGFR2b and EGFR are known to have critical roles in skin repair and inflammation of the skin.”